RCT 200 moderate/severe patients in Thailand, showing significantly lower progression with favipiravir vs. oseltamivir.
NCT04303299 (history).
Study covers favipiravir and
HCQ.
|
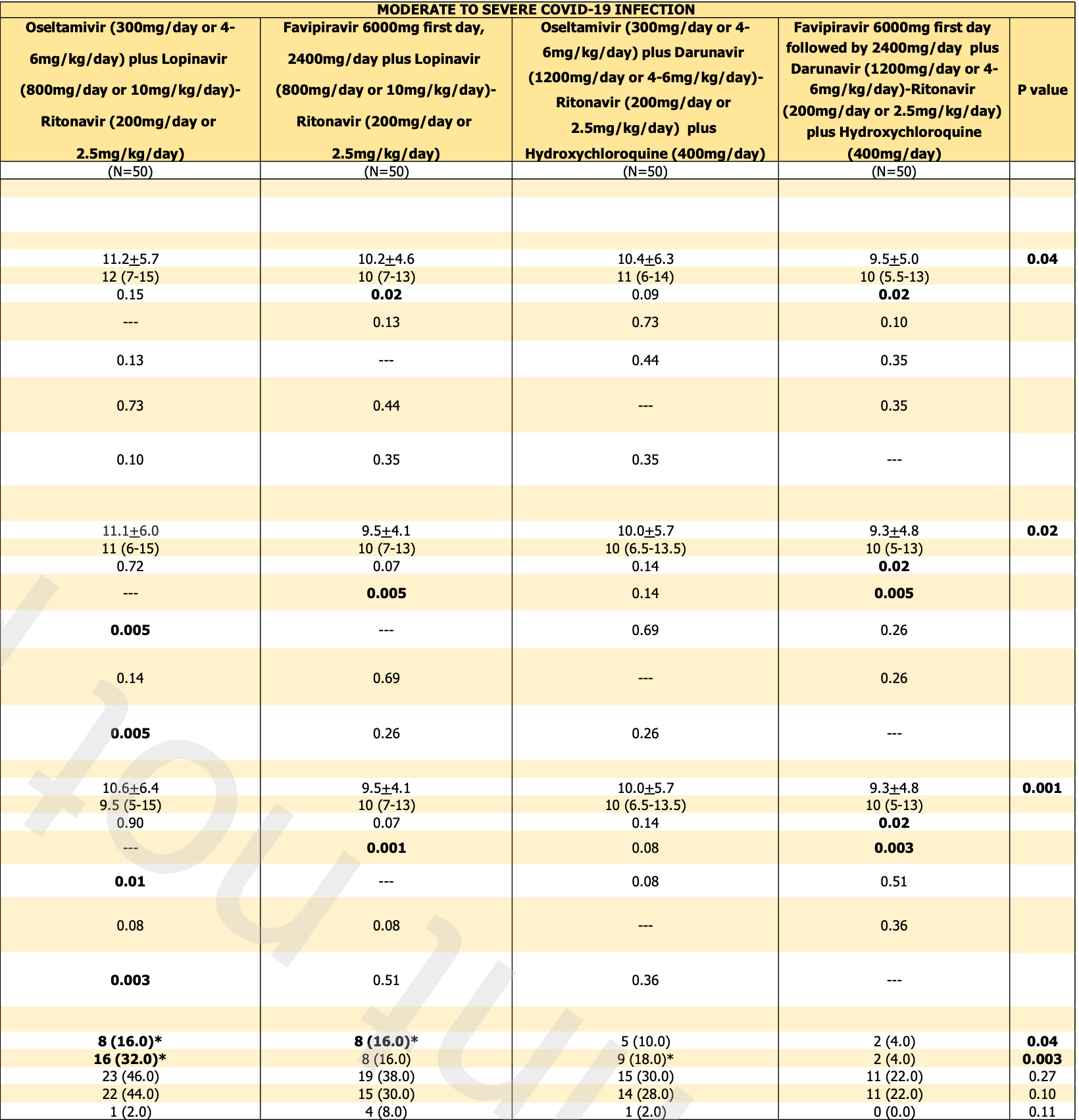
risk of death, 23.1% lower, RR 0.77, p = 0.66, treatment 10 of 100 (10.0%), control 13 of 100 (13.0%), NNT 33, favipiravir arms vs. oseltamivir arms.
|
|
risk of progression, 60.0% lower, RR 0.40, p = 0.009, treatment 10 of 100 (10.0%), control 25 of 100 (25.0%), NNT 6.7, favipiravir arms vs. oseltamivir arms.
|
|
time to viral-, 8.7% lower, relative time 0.91, p = 0.43, treatment mean 9.5 (±5.0) n=50, control mean 10.4 (±6.3) n=50, HCQ arms, primary outcome.
|
|
time to viral-, 8.9% lower, relative time 0.91, p = 0.34, treatment mean 10.2 (±4.6) n=50, control mean 11.2 (±5.7) n=50, non-HCQ arms, primary outcome.
|
|
Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates
|
Atipornwanich et al., 5 Oct 2021, Randomized Controlled Trial, Thailand, peer-reviewed, 16 authors, study period 19 August, 2020 - 28 August, 2021, this trial compares with another treatment - results may be better when compared to placebo, this trial uses multiple treatments in the treatment arm (combined with lopinavir/ritonavir or duranivir/ritonavir/HCQ) - results of individual treatments may vary, trial
NCT04303299 (history) (FIGHT-COVID-19).
M.D Kriangsak Atipornwanich, Associate Professor Subsai Kongsaengdao, M.D Piyathida Harnsomburana, M.D Rienthong Nanna, M.D Chatchawan Chtuparisute, M.D Piamlarp Saengsayan, M.D Kittima Bangpattanasiri, M.D Weerawat Manosuthi, M.D Narumol Sawanpanyalert, M.D Attasit Srisubat, M.D Somchai Thanasithichai, M.D Benchalak Maneeton, M.D Narong Maneeton, Chuthamanee Suthisisang, PhD B Pharm, Jaturong Pratuangdejkul, M.D Somsak Akksilp, Professor Dusit Sujurarat, Hospital Rajavithi, Bangkok Bangkok, Thailand
Various combinations of Favipiravir, Lopinavir-Ritonavir, Darunavir-Ritonavir, high-dose Oseltamivir, and Hydroxychloroquine for the treatment of Covid-19: A randomized controlled trial. (FIGHT-COVID-19 Study)
References
Hata, Koseki, Yamaguchi, Limited inhibitory effects of Oseltamivir and zanamivir on human sialidases, Antimicrob Agents Chemother,
doi:10.1128/AAC.00344-08Hung, Lung, Tso, Triple combination of interferon beta-1b, lopinavirritonavir, and ribavirin in the treatment of patients admitted to hospital with Covid -19: an open-label, randomised, phase 2 trial, Lancet,
doi:10.1016/S0140-6736(20)31042-4Kriangsak, Akksilp, Sawanpanyalert, Srisubat, Thanasithichai et al., Various Combination of Antiviral Treatment of Covid -19 Pneumonia
Wang, Zhang, Du, Remdesivir in adults with severe Covid -19: a randomised, doubleblind, placebo-controlled, multicentre trial [published correction appears in, Lancet